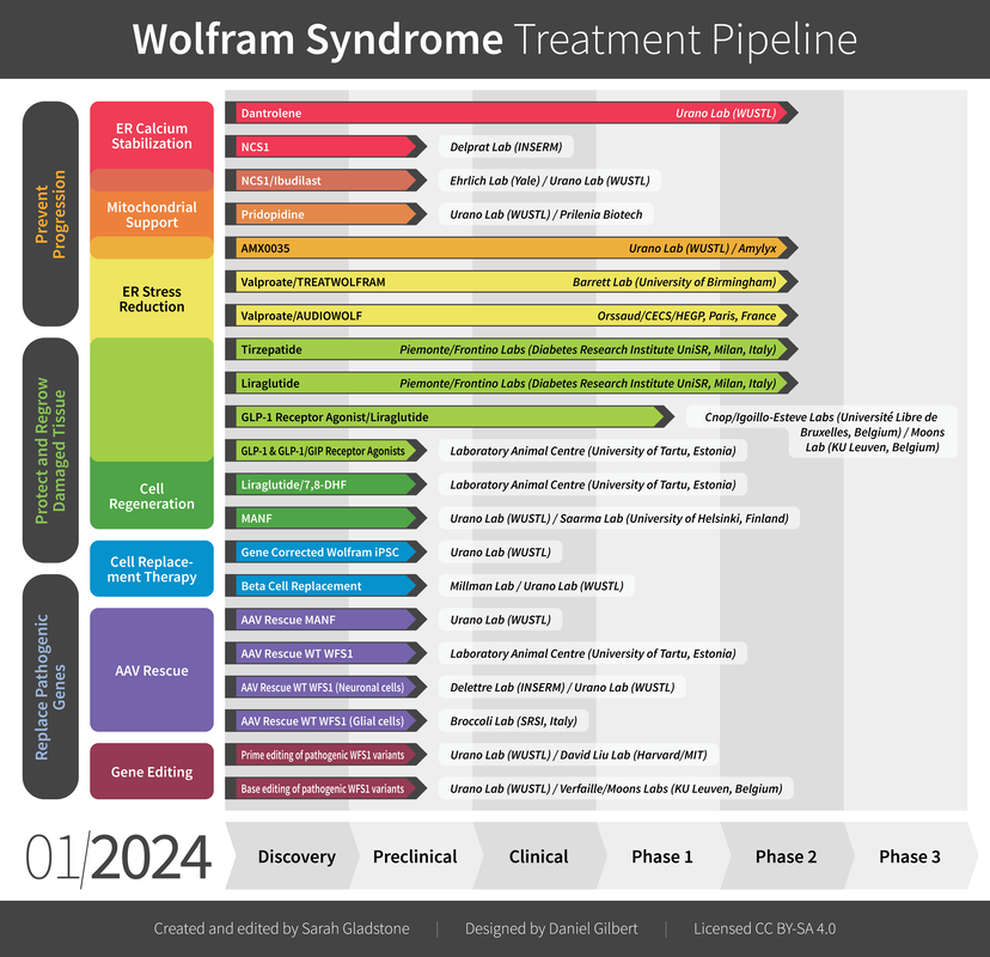
Click each study below for more information
Dantrolene Sodium Clinical Trial - urano
Primary Outcome Measures
The investigators assess the safety and tolerability of dantrolene sodium administered orally at upper end of therapeutic dose range for 6 months in patients with Wolfram syndrome.
Secondary Outcome Measures
• Changes in C-peptide levels in participants assessed by the ELISA assay [ Time Frame: 6 months ]
• Changes in Visual Functioning in participants assessed by Visual Functioning Questionnaire-25. [ Time Frame: 6 months ]
Visual functions will be assessed by Visual Functioning Questionnaire – 25.
• Changes in best-corrected visual acuity in participants measured by Snellen optotype [ Time Frame: 6 months ]
Best-corrected visual acuity will be measured by Snellen optotype. Higher logMar scores indicate worse vision.
• Changes in Neurological Functions in participants assessed by the Wolfram Unified Rating Scale (WURS) [ Time Frame: 6 months ]
Neurological functions will be assessed by the Wolfram Unified Rating Scale (WURS) and standard neurological assessments.
The investigators assess the safety and tolerability of dantrolene sodium administered orally at upper end of therapeutic dose range for 6 months in patients with Wolfram syndrome.
Secondary Outcome Measures
• Changes in C-peptide levels in participants assessed by the ELISA assay [ Time Frame: 6 months ]
• Changes in Visual Functioning in participants assessed by Visual Functioning Questionnaire-25. [ Time Frame: 6 months ]
Visual functions will be assessed by Visual Functioning Questionnaire – 25.
• Changes in best-corrected visual acuity in participants measured by Snellen optotype [ Time Frame: 6 months ]
Best-corrected visual acuity will be measured by Snellen optotype. Higher logMar scores indicate worse vision.
• Changes in Neurological Functions in participants assessed by the Wolfram Unified Rating Scale (WURS) [ Time Frame: 6 months ]
Neurological functions will be assessed by the Wolfram Unified Rating Scale (WURS) and standard neurological assessments.
NCS1
The Delprat team aims to decipher why a deficit in wolframin leads to neurodegeneration. We previously showed that wolframin was essential for the communication between the endoplasmic reticulum and the mitochondria by protecting NCS1 from degradation. NCS1 is a calcium sensor that regulates the endoplasmic reticulum calcium channel IP3R, insuring the proper functioning of the mitochondria, the powerhouse of the cell. Using different complementary methods, we are trying to understand how NCS1 deficit alters cellular physiology in Wolfram syndrome. The ultimate goal is to modulate NCS1 to rescue pathological phenotype.
NCS1/IBUDILAST
Because diabetes mellitus is the first diagnosed symptom of Wolfram syndrome, we aimed to further examine the functions of WFS1 in pancreatic β cells in the context of hyperglycemia. Knockout (KO) of WFS1 in rat insulinoma (INS1) cells impaired calcium homeostasis and protein kinase B/Akt signaling and, subsequently, decreased cell viability and glucose-stimulated insulin secretion. Targeting calcium homeostasis with reexpression of WFS1, overexpression of WFS1’s interacting partner neuronal calcium sensor-1 (NCS1), or treatment with calpain inhibitor and ibudilast reversed deficits observed in WFS1-KO cells. Collectively, our findings provide insight into the disease mechanism of Wolfram syndrome and highlight new targets and drug candidates to facilitate the development of a treatment for this disorder and similar diseases.
Pridopidine - Urano/Prilenia
The research team will use the funds to test the efficacy of a Sigma 1 receptor agonist, pridopidine, developed by Prilenia in pre-clinical models of Wolfram syndrome. Successful completion of this study may lead to a future clinical trial of pridopidine in patients with Wolfram syndrome.
AMX0035 - Urano/amylyx
A common molecular signature of Wolfram syndrome is cellular stress caused by the expression of mutant WFS1 proteins derived from pathogenic WFS1gene variants. Mutant WFS1 proteins tend to misfold in the ER, leading to ER stress. To resolve this protein folding issue, we have been testing chemical chaperones that can optimize the structure of mutant WFS1 proteins. In collaboration with Amylyx, a biotech company in Cambridge, MA, Urano Lab and Washington University in St. Louis have completed recruitment for the phase II trial of AMX0035 and the trial is underway. AMX0035 is a combination therapy designed to reduce neuronal death through blockade of key cellular death pathways originating in the mitochondria and endoplasmic reticulum (ER). This clinical trial is designed to demonstrate that treatment is safe, tolerable, and to evaluate the effect of AMX0035 on residual beta cell functions by monitoring c-peptide levels during a 0-240 minute mixed-meal tolerance test. The trial will also assess the effects of AMX0035 on changes to diabetic measurements including daily insulin dose, time in good glucose range, and HbA1c levels. Effect on best-corrected visual acuity in both eyes will also be evaluated.
VALPROATE/TREATWOLFRAM - Barrett
This phase II clinical trial is a randomised, double-blind, placebo-controlled 3 year intervention Trial in 70 patients with Classical Wolfram Syndrome aged 5 years and over. The primary outcomes of the Trial are considered to be clinically relevant and of sufficient magnitude to be beneficial, as a successful Trial outcome will mean that patients will retain a clinically useful degree of visual acuity and it will decline at a slower rate than in the untreated patients.
Valproate/AUDIOWOLF
AUDIOWOLF: A phase II, open-label, efficacy study of daily administration of sodium valproate in patients clinically affected by Wolfram syndrome due to monogenic mutation.
AUDIOWOLF trial
AUDIOWOLF trial
Tirzepatide - PIEMONTE/FRONTINO
Towards personalized precision medicine in rare disease: tirzepatide (a dual glucose dependent insulinotropic polypeptide and glucagon-like peptide-I receptor agonist) monotherapy in patients with Wolfram syndrome type 1.
Tirzepatide, a new drug for type 2 diabetes mellitus and obesity, as a possible treatment to modify the clinical evolution of Wolfram syndrome type 1
Tirzepatide, a new drug for type 2 diabetes mellitus and obesity, as a possible treatment to modify the clinical evolution of Wolfram syndrome type 1
Liraglutide - Piemonte/Frontino
GLP-1 Receptor Agonist/Liraglutide - Belgium
Cnop/Igoillo-Esteve labs
GLP-1 analogs, such as liraglutide and dulaglutide among others, are used to treat type 2 diabetes. These drugs are known to promote pancreatic beta cell function and survival, may cross the blood brain barrier, and might have potential beneficial effect on neurons and retinal cells. GLP-1 analogs may therefore be of interest in Wolfram syndrome, to prevent or treat diabetes and potentially neurodegeneration.
We have established preclinical models of Wolfram syndrome to test GLP-1 analogs. These models include WFS1 knockout mice, a human beta cell line in which WFS1 is silenced, and induced pluripotent stem cells (iPSC) from people with Wolfram syndrome; these iPSCs are differentiated into beta cells and cerebellar neurons.
Our preclinical data indicate that GLP-1 analogs prevent and reverse diabetes in Wolfram syndrome mice, and improve the function and survival of WFS1-deficient human beta cells and iPSC-derived beta cells from patients with Wolfram syndrome. The effect of GLP-1 analogs on iPSC-derived neurons is still under study. In collaboration with Prof. Lieven Moons, KULeuven, Belgium, the impact of GLP-1 analogs on vision of Wolfram syndrome mice is being investigated.
Based on the preliminary data, liraglutide was started (off-label use) in two 9-year-old children with diabetes and Wolfram syndrome. Liraglutide lowered their sugar levels and reduced glycemic variability, and it reduced the amount of insulin needed by 40 to 75%.
We have established preclinical models of Wolfram syndrome to test GLP-1 analogs. These models include WFS1 knockout mice, a human beta cell line in which WFS1 is silenced, and induced pluripotent stem cells (iPSC) from people with Wolfram syndrome; these iPSCs are differentiated into beta cells and cerebellar neurons.
Our preclinical data indicate that GLP-1 analogs prevent and reverse diabetes in Wolfram syndrome mice, and improve the function and survival of WFS1-deficient human beta cells and iPSC-derived beta cells from patients with Wolfram syndrome. The effect of GLP-1 analogs on iPSC-derived neurons is still under study. In collaboration with Prof. Lieven Moons, KULeuven, Belgium, the impact of GLP-1 analogs on vision of Wolfram syndrome mice is being investigated.
Based on the preliminary data, liraglutide was started (off-label use) in two 9-year-old children with diabetes and Wolfram syndrome. Liraglutide lowered their sugar levels and reduced glycemic variability, and it reduced the amount of insulin needed by 40 to 75%.
Moons Lab
The Neural Circuit Development and Regeneration research group at the University of Leuven (Biology Department, KU Leuven, Belgium), led by Prof. L. Moons and Dr. Lies De Groef, aims to define the cellular and molecular mechanisms underlying neurodegeneration, -inflammation and -regeneration in the injured, diseased or aged central nervous system. Within their research, they focus on the inter-relatedness of neurobiology and ophthalmology research, and position the eye ‘as a window to the brain’. Besides glaucoma, Alzheimer’s and Parkinson’s disease, they have a major interest in Wolfram syndrome. Via ocular and MRI imaging, electrophysiology and visual function testing in laboratory animals, they try to unravel the impact of Wolfram syndrome on the retina and visual system and understand the underlying disease mechanisms. Their studies in a Wolfram mouse model (Wfs1Δexon8) revealed progressive vision loss, neuronal dysfunction and neuroinflammation in the retina, and axonal conduction defects in the optic nerve. Based on these findings and their special interest in the role of glial cells (oligodendrocytes, micro- and astroglia) in the pathogenesis of Wolfram syndrome, they are further complementing this work with mechanistic studies in patient iPSC-derived cells (in collaboration with Prof. C. Verfaillie and G. Bultynck, KU Leuven). Furthermore, building on their expertise in visual system phenotyping, the Neural Circuit Development and Regeneration research group is also evaluating the effect of novel therapeutics to prevent blindness, including preclinical studies with GLP-1 analogues (in collaboration with M. Igoillo Esteve, Université Libre de Bruxelles) and gene editing approaches (in collaboration with C. Verfaillie).
GLP-1&GLP-1/GIP Receptor Agonists - Plaas/Estonia
Liraglutide/7,8 DHF
University of Tartu, Laboratory Animal Center - Estonia
Liraglutide, 7,8-DHF and their co-treatment prevents loss of vision and cognitive decline in a Wolfram syndrome rat model. Kadri Seppa, Toomas Jagomäe, Kaia Grete Kukker, Riin Reimets,Marko Pastak, Eero Vasar, Anton Terasmaa & Mario Plaas. Sci Rep11, 2275 (Jan 26, 2021).
Wolfram syndrome (WS) is a monogenic progressive neurodegenerative disease and is characterized by various neurological symptoms, such as optic nerve atrophy, loss of vision, cognitive decline, memory impairment, and learning difficulties. GLP1 receptor agonist liraglutide and BDNF mimetic 7,8-dihydroxyflavone (7,8-DHF) have had protective effect to visual pathway and to learning and memory in different rat models of neurodegenerative disorders. Although synergistic co-treatment effect has not been reported before and therefore the aim of the current study was to investigate liraglutide, 7,8-DHF and most importantly for the first time their co-treatment effect on degenerative processes in WS rat model. We took 9 months old WS rats and their wild-type (WT) control animals and treated them daily with liraglutide, 7,8-DHF or with the combination of liraglutide and 7,8-DHF up to the age of 12.5 months (n = 47, 5–8 per group). We found that liraglutide, 7,8-DHF and their co-treatment all prevented lateral ventricle enlargement, improved learning in Morris Water maze, reduced neuronal inflammation, delayed the progression of optic nerve atrophy, had remyelinating effect on optic nerve and thereby improved visual acuity in WS rats compared to WT controls. Thus, the use of the liraglutide, 7,8-DHF and their co-treatment could potentially be used as a therapeutic intervention to induce neuroprotection or even neuronal regeneration.
MANF - Urano
Given the deleterious effects of chronic ER stress on specific cell types in Wolfram syndrome, there is a need for regenerative medicine efforts aimed at replacing these damaged tissues. More specifically, there is a need for replacing insulin-producing pancreatic b-cells and retinal ganglion cells in patients, as defects in these cell types have the greatest impact on patients’ quality of life. To this end, we have been developing and testing regenerative therapy options using induced a secretory survival factor termed mesencephalic astrocyte-derived neurotrophic factor (MANF). Preclinical studies in a mouse model of Wolfram syndrome show that MANF (recombinant MANF peptide and AAV expressing MANF) can activate the proliferation of remaining bcells. We are currently testing the efficacy of MANF on regeneration of retinal ganglion cells in a mouse model of Wolfram syndrome.
Gene Corrected Wolfram iPSC
We have been developing regenerative therapy in combination with gene correction using induced pluripotent stem cells (iPSCs). We have generated iPSCs from patients with Wolfram syndrome and then correcting WFS1 gene mutations by CRISPR/CAS9. These cells have then been differentiated into pancreatic b-cells and then transplanted into diabetic mice. We could cure diabetes in these mice using bcells differentiated from gene corrected iPSCs derived from Wolfrma syndrome patients. We are currently assessing the functional restoration of retinal ganglion cells differentiated from these gene-corrected iPSCs of Wolfram syndrome patients.
Beta Cell Replacement
Use of the eye cells, brain cells, and insulin-producing pancreatic cells differentiated from Wolfram syndrome patient-derived iPSCs would provide a source of autologous replacement cells We corrected pathogenic gene variants causing Wolfram syndrome in iPSCs using CRISPR/Cas9 gene editing, and differentiated them to insulin-producing pancreatic beta cells. We could successfully restore beta cell functions with this strategy and cure diabetes in mice. We are currently testing this strategy using other types of cells, including iPSC-derived retinal cells and brain cells.
AAV Rescue MANF
Given the deleterious effects of chronic ER stress on specific cell types in Wolfram syndrome, there is a need for regenerative medicine efforts aimed at replacing these damaged tissues. More specifically, there is a need for replacing insulin-producing pancreatic b-cells and retinal ganglion cells in patients, as defects in these cell types have the greatest impact on patients’ quality of life. To this end, we have been developing and testing regenerative therapy options using induced a secretory survival factor termed mesencephalic astrocyte-derived neurotrophic factor (MANF). Preclinical studies in a mouse model of Wolfram syndrome show that MANF (recombinant MANF peptide and AAV expressing MANF) can activate the proliferation of remaining bcells. We are currently testing the efficacy of MANF on regeneration of retinal ganglion cells in a mouse model of Wolfram syndrome.
AAV Rescue with WT WFS1 - Plaas/Tartu
AAV Rescue WT WFS1 (Neuronal/retinal Cells) Delettre/Urano
Our ultimate goal for Wolfram syndrome is to provide a cure by gene therapy. Our first approach towards achieving this goal is through gene transfer. Using AAV sytems, we plan to transfer wildtype WFS1 into the retinal cells of patients with Wolfram syndrome, an approach that has proven successful in the treatment of retinitis pigmentosa. Preclinical studies in mouse and rat models of Wolfram syndrome are currently being elaborated in our lab.
AAV Rescue WT WFS1 (Glial Cells) - Broccoli lab
Prime Editing of Pathogenic WFS1 Variants - Urano/Liu
The root cause of Wolfram syndrome is pathogenic changes in the WFS1 gene. Therefore, correcting these changes in the WFS1 gene is the best way to treat Wolfram syndrome. We initially used the original CRISPR-Cas9 gene editing technology and then used a newer technology, base editing. Recently, we have started a collaboration with Dr. David Liu’s group at Harvard/MIT (https://www.liugroup.us/) and started using Prime Editing to correct WFS1 gene pathogenic changes in Wolfram syndrome because of safety reasons. Prime Editing is a newer version of gene editing, considered the best gene editing technology available to date. We can now test the technology in high-quality Wolfram syndrome iPS cells and iPSC-derived retinal ganglion cells to develop gene therapy for optic nerve atrophy. We are also creating cortical neurons (brain cells) and inner hair cells (ear cells) from Wolfram iPSCs for developing gene therapy for neurodegeneration and hearing loss.
Furthermore, we created rodent models with pathogenic changes in the Wfs1 gene to test Prime Editing in vivo. We call them humanized Wolfram mice and Wolfram rats. Our ultimate goal is to use this therapeutic modality for our patients.
Furthermore, we created rodent models with pathogenic changes in the Wfs1 gene to test Prime Editing in vivo. We call them humanized Wolfram mice and Wolfram rats. Our ultimate goal is to use this therapeutic modality for our patients.
Base Editing of Pathogenic WFS1 Variants - Verfaille/Moons/Urano
Although rare individually, genetic disorders collectively constitute a common health problem. As the cause of these diseases is a defective gene, gene therapy would be able to resolve all of these disorders. Wolfram syndrome is a genetic disorder, with the ultimate symptoms of Diabetes, blindness and deafness in young kids. The most recent method of gene therapy is gene editing. Gene editing is repairing the defect of the gene by using molecular scissors such as CRISPR/Cas9. CRISPR/Cas9, which is the most commonly used type of this system is, composed of two components Cas9 protein which can make a cleavage in DNA and a guide RNA that is a RNA molecule which binds to Cas9 and guide it towards a specific target sequence of 20 bases. CRISPR/Cas9 allows us to cut the human genome by generating DNA double-strand breaks (DSB) close to the mutation. Such DSB is sensed by two main cellular repair pathways, nonhomologous end-joining (NHEJ) and homology-directed repair (HDR). While NHEJ is generally known to be an error-prone pathway causing insertions and deletions (indels) of DNA bases, which occurs independently of a repair template, HDR relies on the presence of a repair template to refill the correct bases and is considered an error-free pathway. Therefore, HDR has been extensively tested and used for precise gene editing. Given that HDR is restricted to the S and G2 phases of the cell cycle, only present in dividing cells, this approach might not be suitable for editing and repairing the genome in non-dividing cells. However many of monogenic disorders, including Wolfram syndrome cause disease, because the function of terminally differentiated non-dividing cells in different organs is impaired. Therefore, to reverse the defect caused by monogenic diseases, therapies that can correct the genetic defect in non-dividing cells are required. One very recent tool that is made based on CRISPR/Cas9 system is called Base editor. Base editing is a direct replacement of a single DNA base with the correct one without making a DSB and therefore not relying on HDR. Base editor (BE) is an engineered fusion enzyme consist of Cas9 and cytidine deaminase (CD) that enables a C-G to T-A conversion in an activity window that can be as narrow as 1-2 bases. Since the initial description, BE has been extensively improved and expanded. Another important base editing tool is adenine base editors (ABEs) contains a DNA adenosine deaminase instead of CD and enables a T-A to C-G transition. ABE is a major milestone in base editing considering that the target mutations (C-G to T-A ) account for half of the pathogenic known point mutations in human. Some of the mutations causing wolfram syndrome are targetable by ABE (e.g. c.2002 C>T or c.1620 G>A). ABE has been proven effective in mice on different non-dividing cell types including the cells in retina. However, due to differences between mice and human in many aspects and specifically on DNA repair system, before moving forward to clinical application of ABE, it is important to evaluate this tool on human cells in vitro (in a dish). Therefore, in our study we are planning to evaluate the efficiency of ABE on human derived non-dividing cells. Since these cells are not accessible from human, we will generate them in the lab from Wolfram patient derived stem cells. We are focused on two cell types; oligodendrocytes and retinal ganglion cells as two non-dividing cells leading to eye and brain related symptoms of Wolfram syndrome. We successfully differentiated our patient derived stem cells to oligodendrocytes and retinal ganglion cells. Next, we tested the efficiency of the ABE tool on the stem cells of patients to confirm that the tool is generated correctly. Our result showed highly efficient correction of the mutated DNA base in the stem cells of patients (around 90%). Therefore, we proceed with making delivery vectors to be able to get the ABE tool to oligodendrocyte and retinal ganglion cells. Our vectors are successfully generated, and we are currently performing delivery of the tools to our target cells. We hope to release the result of this part in near future.
–Catherine Verfaille
–Catherine Verfaille
Prof. C. Verfaillie and G. Bultynck, KU Leuven are working in collaboration with The Neural Circuit Development and Regeneration research group at the University of Leuven (Prof. L. Moons and Dr. Lies De Groef) on mechanistic studies in patient iPSC-derived cells. The Verfaillie lab is collaborating with M. Igoillo Esteve, (Université Libre de Bruxelles) and the Moons lab evaluating the effect of novel therapeutics to prevent blindness, includng preclinical studies of gene editing approaches.